What is a Vibratome (Vibrating Microtome)?
A vibratome is a type of microtome (vibrating microtome) used to section biological tissue using a vibrating stainless steel or ceramic blade. Unlike rotary microtomes, cryostats, or paraffin-embedded slicing methods, a vibratome allows for the preparation of unfixed or lightly fixed tissue sections without the need for freezing or embedding in wax. Vibratomes are designed to produce thick, viable slices of tissue—typically between 20 and 1000 microns—while preserving structural and cellular integrity. This makes them especially valuable for neuroscience, electrophysiology, pharmacology, and tissue culture applications where cell viability and accurate morphology are critical.
Compare models and Request a Quotation

9000SMZ
Fully automated system for high-throughput, precise slicing.
Blade Frequency: 20 - 120 Hz
Blade Amplitude: 0.5 - 3.0 mm
Advance Speed Resolution: 0.001 mm/s

5100mz-Plus
Enhanced vibration stability for live tissue applications.
Blade Frequency: 50 - 80 Hz
Blade Amplitude: 0.5 - 1.5 mm
Advance Speed Resolution: 0.01 mm/s

5100mz
Reliable manual operation ideal for routine lab slicing.
Blade Frequency: 50 - 80 Hz
Blade Amplitude: 0.5 - 1.5 mm
Advance Speed Resolution: 0.01 mm/s
How Vibrating Microtomes Work: Live and Fixed Tissue Sectioning
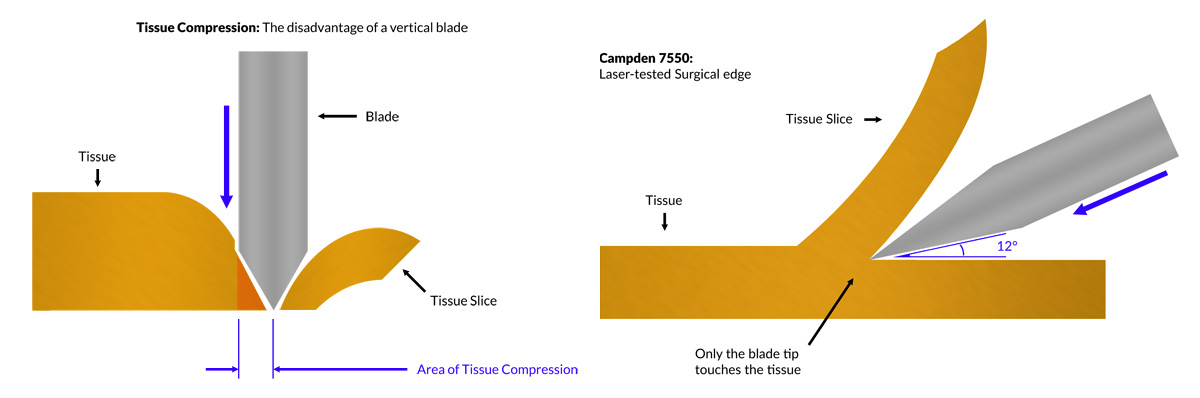
The core principle of a vibratome is the use of a high-frequency vibrating blade that oscillates horizontally while slowly advancing through a tissue sample. The vibration minimizes shearing forces and reduces mechanical damage, allowing researchers to obtain smooth and consistent slices even from soft or delicate tissues like brain, liver, or spinal cord. Horizontal sectioning ensures there is no compression to the underlying tissue; vertical sectioning would introduce compression of, and consequently damage, the tissue.
Vibratomes typically include:
- A motorized vibrating blade holder
- A specimen stage or holder to secure tissue samples
- A buffer tray or bath filled with solutions like aCSF or PBS
- Adjustable settings for vibration amplitude, frequency, advance speed and section thickness
In research labs, vibratomes are used to prepare organotypic brain slices, tissue explants, and samples for imaging, immunohistochemistry, or functional assays. Because vibratomes do not require freezing, they are ideal for preserving tissue function and morphology during slicing.
Common synonyms include vibrating microtome and vibrating blade microtome.
Applications of Vibratomes in Neuroscience, Pathology, and Life Science
Vibratomes are used across a wide range of scientific disciplines where thick, intact tissue slices are needed without the structural distortion caused by traditional microtomy or cryostat sectioning. Because vibratomes preserve cell viability and tissue architecture, they are ideal for functional and structural studies alike.
🧠 Neuroscience
Vibratomes are the gold standard for slicing live brain tissue in neuroscience research. They enable the preparation of organotypic slices used in:
- Electrophysiology (e.g., patch clamp, field potential recordings)
- Calcium imaging and voltage-sensitive dye studies
- Optogenetics and neural circuit mapping
- Brain slice cultures for long-term studies
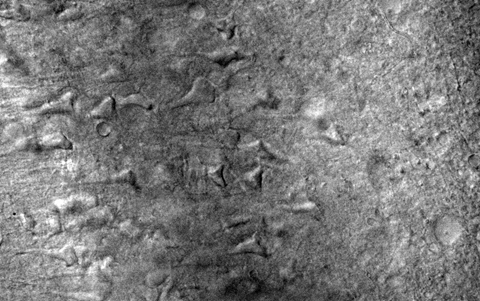
Entorhinal Cortex Slice from 9 month-old Rat
The ability to cut fresh brain sections while maintaining synaptic integrity makes vibratomes indispensable in cognitive, behavioral, and pharmacological neuroscience.
🧪 Pharmacology & Toxicology
In pharmacological studies, vibratomes are used to section soft tissue like liver, kidney, and intestinal mucosa to assess:
- Drug absorption and diffusion rates
- Tissue response to compound exposure
- Metabolic enzyme activity and bioavailability
Vibratome-cut tissue can also be used in ex vivo toxicology models and precision-cut tissue slice (PCTS) systems.
🔬 Pathology & Histology
While not a replacement for paraffin sectioning, vibratomes are widely used in histology for:
- Immunohistochemistry (IHC) on thick sections
- Fluorescent microscopy of fixed samples
- Preparing tissue for 3D imaging and clearing techniques
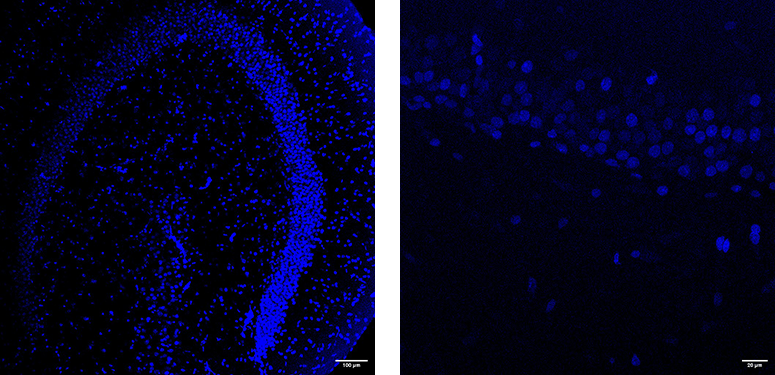
DAPI-stained 350µm hippocampal slices taken from a male Wistar rat of approximately 2 months old. Slicing parameters were ceramic blade; blade frequency 50Hz; blade amplitude 1mm; advance speed 0.05mm/s. Tissue was sliced fresh and then post-fixed in 4% formaldehyde PBS for 24 hours, then stained with Vectashield-DAPI mountant before imaging on a Leica SP8 confocal.
Researchers working on antibody labeling or spatial biology benefit from the thick sections vibratomes produce, which allow better penetration of stains and dyes.
🧫 Tissue Engineering & Regenerative Medicine
In tissue engineering, vibratomes assist in creating standardized tissue slices for:
- Scaffold seeding and decellularization studies
- Biomaterial-tissue interface modeling
- Mechanical testing of soft tissue composites
- Creating living myocardial slices for cardiac electrophysiology and regenerative medicine
These applications benefit from vibratome precision and the ability to preserve tissue viability. Living myocardial slices, in particular, are gaining prominence in translational cardiology for studying heart function, drug responses, and tissue regeneration under near-native conditions.
📷 Microscopy & Imaging
Vibratomes produce smooth surfaces ideal for high-resolution imaging, including:
- Confocal and multiphoton microscopy
- CLARITY and tissue clearing techniques
- 3D reconstruction from serial sections
Vibratome vs. Cryostat vs. Traditional Microtome: What’s the Difference?
Not all microtomes are created equal. Depending on your application—whether it’s live tissue slicing, routine histology, or frozen sectioning—each type of microtome has distinct advantages and limitations. Below is a detailed comparison of the three most common types:
Key Differences Between Microtome Types
- Vibratome: Ideal for live or fixed tissue without the need for freezing or embedding. Enables thicker, viable slices.
- Rotary Microtome: Standard for thin paraffin-embedded tissue sections. Excellent for routine histology.
- Cryostat: Used for quick-frozen tissue slices in clinical and pathology labs. Ideal for rapid diagnostics.
Use the table below to compare their core features.
| Feature | Vibratome | Rotary Microtome | Cryostat |
|---|---|---|---|
| Tissue Type | Live or fixed | Paraffin-embedded | Frozen |
| Slice Thickness | 20–1000 µm | 1–20 µm | 5–40 µm |
| Sample Preparation | No freezing/embedding | Paraffin block required | Rapid freezing needed |
| Ideal Use Case | Electrophysiology, tissue culture | Routine histopathology | Rapid intra-op diagnostics |
| Section Quality | High for thick soft tissue | Very fine detail for thin cuts | Good, but potential freeze artifacts |
| User Skill Level | Moderate | Moderate | Advanced |
| Required Equipment | Vibrating microtome + buffer bath | Rotary microtome | Cryostat chamber |
| Cell Viability | Excellent | Lost during processing | Low (frozen stress) |
| Common Applications | Neuroscience, regenerative medicine | Histology, pathology labs | Clinical biopsy review |
Choosing the Best Vibratome for Your Research Lab
Selecting the right vibratome depends on several factors including the type of tissue you work with, your research goals, and your lab's budget and workflow needs. Below is a practical guide to help you evaluate which model is best suited to your application.
🔬 Tissue Type and Sample Size
Consider the types of tissues you'll be sectioning most often:
- Brain, spinal cord, and neural tissue: Require gentle slicing with minimal compression. Models with minimal blade z-axis deflection, adjustable amplitude and blade frequency, and an advance speed resolution of 0.01mm/s are ideal.
- Myocardial, liver, or kidney slices: Require a powerful blade mechanism and flexible tissue mount configurations.
- Fixed vs. fresh tissue: Fixed samples are easier to cut, but live slices demand precise vibration control to preserve viability.
⚙️ Key Performance Specifications
Match the vibratome's capabilities to your experimental needs:
- Slice thickness range: Ensure your model supports your required range (e.g. 20-1000 µm).
- Blade frequency and amplitude: Higher frequency vibration improves section quality in older or more fibrous tissue; slower blade frequencies are recommended for softer tissues. Read this Blade Article.
- Precision and repeatability: Look for micrometer accuracy and programmable settings if consistency is critical.
- Z-Axis deflection. Changing a vibratome blade always introduces blade Z-axis deflection. For demanding applications on live tissue, it is, therefore, important that you are able to measure the Z-axis deflection at the blade edge, and then be able to correct it. There is no automatic way of achieving this (see Geiger, J., Bischofberger, J., Vida, I. et al. Patch-clamp recording in brain slices with improved slicer technology. Pflügers Arch - Eur J Physiol 443, 491–501 (2002). https://doi.org/10.1007/s00424-001-0735-3).
🤖 Automation & User Interface
Labs with high throughput or multiple users often benefit from:
- Automated slicing modes: Useful for repeatable workflows
- Programmable memory: Store and recall frequently used settings
- Digital readouts: Easier calibration and monitoring
Manual vibratomes are cost-effective but require more user skill. Consider user experience and training level when choosing.
💼 Budget and Lab Resources
Not all labs need top-tier models. Here's how to align budget with function:
- Entry-level models: Ideal for teaching labs or simple fixed tissue slicing
- Mid-range models: Good for routine histology and some live tissue work
- High-performance models: Designed for demanding neuroscience and tissue viability studies, e.g. for visual patch clamp or calcium imaging.
Always factor in accessory costs (blades, trays, bath inserts) and support/service availability.
Take a look at our top models like the 9000SMZ and 5100mz.
📩 Still Unsure?
Our application specialists are happy to help. Contact our technical team to discuss your use case and we?ll recommend the best vibratome for your workflow.
Vibratome Maintenance & Setup
To maintain vibratome performance:
- Potentially use fresh blades. Cermaic blades can last many weeks, so just ensure they remain clean.
- For sectioning live tissue for demanding applications, measure and correct the Z-axis deflection of the blade.
- Keep tissue submerged in buffer
- Clean device after each use
Vibratome Setup & Demo Video
Frequently Frequently Asked Questions About Vibrating Microtomes
- Can vibratomes or vibrating microtomes cut live brain tissue?
- Yes. Vibratomes are widely used to cut live, unfixed brain, heart, lung, liver slices for electrophysiology, imaging, and culture. Their low-vibration motion helps preserve synaptic and structural integrity during slicing.
- Do I need to fix tissue before slicing with a vibratome or vibrating microtome?
- No. Vibratomes are designed to cut both fixed and fresh tissue. For soft tissues like brain or liver, slicing without fixation is common in live experiments. For histological staining, fixed tissues may be preferred.
- What slice thicknesses can vibratomes or vibrating microtomes achieve?
- Most vibratomes support a slice thickness range of 20 to 1000 microns. Common applications include 300–500 µm for electrophysiology and 50–100 µm for imaging or staining.
- What tissues can I cut with a vibratome or vibrating microtome?
- Common tissues include brain, spinal cord, liver, kidney, lung, heart (for living myocardial slices), and organoids. The key is that the tissue is soft and can be embedded or secured properly for slicing.
- Are vibratome blades reusable?
- Yes. Stainless steel blades and ceramic blades can be re-used. Stainless steel blades can be used for a few days. Ceramic blades can last many weeks. Blade quality directly affects slice smoothness and cellular preservation. Razor blades should NEVER be used as they are designed to cut hair and not skin and are, therefore, relatively blunt. Read this article on blade choice and hardness.
- Do I need a special embedding medium?
- No wax or freezing is required. Tissues can be embedded in low-melting agarose to provide support during slicing, however the user should be conscious of the time to do this and whether the tissue will receive adequate oxygenation. The agarose block is then mounted in a buffer tray such as aCSF or PBS. Alternatively users mount the tissue and mount an agar bloack behind the tissue to provide additional support.
- What buffer should I use during slicing?
- Most neuroscience and live tissue applications use oxygenated artificial cerebrospinal fluid (aCSF). For fixed tissues, phosphate-buffered saline (PBS) is common. Buffer keeps tissue hydrated and stable.
- Can vibratomes be used for fixed paraffin-embedded tissues?
- No. Paraffin-embedded tissues are best sliced with a rotary microtome. Vibratomes are optimised for slicing unembedded, soft tissues without dehydration or embedding processes.
- Are vibratomes difficult to calibrate?
- No. Most modern vibratomes include digital controls for slice thickness, vibration frequency, and blade advance..
- What accessories do I need?
- Common accessories include blade holders, specimen clamps, buffer trays, chilling modules (optional), and spare blades. Some labs also use LED lighting or microscope mounts for real-time viewing.
Why Choose Campden Instruments for your Vibratome?
Campden Instruments is a global leader in precision tissue slicing solutions, trusted by researchers in neuroscience, histology, pharmacology, and biomedical engineering for over 40 years. We don’t just manufacture instruments—we collaborate with scientists to solve real-world research challenges.
🧬 Proven Performance in Research Labs
Our vibratomes are used by leading institutions including the University of Oxford, Harvard Medical School, the Max Planck Institute, and many others. Campden’s instruments are frequently cited in peer-reviewed publications for their reliability and precision in preparing live tissue samples.
We have developed models tailored to meet the demands of:
- Advanced neuroscience (e.g., electrophysiology, calcium imaging)
- Cardiovascular studies (e.g., living myocardial slices)
- Routine histopathology and teaching labs
⚙️ Innovation That Matters
Campden was the first to offer:
- Fully programmable vibratome systems with memory presets
- Ultra-low blade z-axis deflection blade holders for ultra-smooth slicing
- A range of blade frequencies, amplitudes, precision advance speed and choice of blades.
Every feature we design is engineered with the end user in mind—whether you're preparing delicate brain slices or durable cardiac tissue.
🌍 Global Support Network
Campden products are supported by an international network of technical reps and authorised distributors. We provide:
- Remote and in-person training for lab staff
- Fast global shipping and customs-ready packaging
- Direct access to technical support and service specialists
We’re known not just for the quality of our instruments, but the responsiveness of our people.
🔒 Built for Longevity and Accuracy
Every Campden vibratome is manufactured under ISO-certified quality standards, using high-grade components built for consistent, repeatable performance over years of lab use.
Our instruments are:
- Calibrated and QC-tested before shipment
- Backed by industry-leading warranties
- Designed to be serviceable with readily available parts
💡 Let’s Build Your Ideal Slicing Setup
Whether you're upgrading your neuroscience core facility or starting a teaching lab, Campden Instruments has a vibratome solution tailored to your needs. Contact our team to discuss your application, and we'll help you configure the ideal system—blade holders, trays, and accessories included.
Customer Spotlight
Talking Science with our vibratome users
Compare Campden Vibratome Models: Specs, Slice Thickness, and Features

9000SMZ
Fully automated system for high-throughput, precise slicing.
Blade Frequency: 20 - 120 Hz
Blade Amplitude: 0.5 - 3.0 mm
Advance Speed Resolution: 0.001 mm/s

5100mz-Plus
Enhanced vibration stability for live tissue applications.
Blade Frequency: 50 - 80 Hz
Blade Amplitude: 0.5 - 1.5 mm
Advance Speed Resolution: 0.01 mm/s

5100mz
Reliable manual operation ideal for routine lab slicing.
Blade Frequency: 50 - 80 Hz
Blade Amplitude: 0.5 - 1.5 mm
Advance Speed Resolution: 0.01 mm/s
Need help choosing? Contact our technical team
Publications & Research Papers
Here are a few select references for different tissues. A complete bibliography is available for all tissues here.
Precision Cut Lung Slices
Fastiggi, V. A., Mank, M. M., Caporizzo, M. A., & Poynter, M. E. (2025). Beta-Hydroxybutyrate Inhibits Bronchial Smooth Muscle Contraction. bioRxiv, 2025-02. https://doi.org/10.1101/2025.02.24.639075
Melo-Narvaez, M.C., Gölitz, F., Jain, E. et al. (2025). Cold storage of human precision-cut lung slices in TiProtec preserves cellular composition and transcriptional responses and enables on-demand mechanistic studies. Respir Res 26, 57. https://doi.org/10.1186/s12931-025-03132-w
Cardiac Slices
Abbas, N., Bentele, M., Waleczek, F. J., Fuchs, M., Just, A., Pfanne, A., ... & Thum, T. (2024). Ex vivo modelling of cardiac injury identifies ferroptosis-related pathways as a potential therapeutic avenue for translational medicine. Journal of molecular and cellular cardiology, 196, 125-140. https://doi.org/10.1016/j.yjmcc.2024.09.012
Jordan, M., Schmieder, F., Waleczek, F. J., Polk, C., Stucki-Koch, A., Philipp, J., ... & Fiedler, J. (2024). De novo establishment of an ex vivo culture for living myocardial slices applying a microphysiological system–MPSlms. Current Directions in Biomedical Engineering (Vol. 10, No. 4, pp. 347-350). https://doi.org/10.1515/cdbme-2024-2085




