The vibratome has emerged as a critical tool in organoid research, enabling precise tissue sectioning that preserves delicate three-dimensional cytoarchitecture for both structural and functional studies
The vibratome has emerged as a critical tool in organoid research, enabling precise tissue sectioning that preserves delicate three-dimensional cytoarchitecture for both structural and functional studies. Achieving consistent, high-quality sections is essential for reliable organoid research. Our advanced vibratome technology is designed to slice delicate organoids with exceptional accuracy, preserving cell structure and viability for downstream analysis. Whether you are working in neuroscience, developmental biology, or disease modelling, a vibratome offers the control and precision needed to prepare organoid samples without causing mechanical damage.
Two recent investigations — Hong et al. (2022) and Fagerlund et al. (2022) — illustrate complementary roles of vibratome slicing in cerebral organoid workflows: histological characterisation and advanced electrophysiology.
In their optimised protocol for generating cerebral organoids from human embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs), Hong and colleagues used a vibratome (Campden 7000 SMZ) to section fixed, agarose-embedded organoids.
Sectioning Parameters: 50–100 µm thickness; cutting speed 0.3 mm/s; amplitude 1.00 mm; frequency 50 Hz in PBS.
Purpose: Thicker sections facilitated 3D immunocytochemistry, maintaining cortical and ventricular structures for confocal microscopy.
Outcome: Enabled high-resolution mapping of cortical plate, ventricular zones, and rosette structures without structural collapse seen in thinner cryosections.
Integration with Clearing: Vibratome-prepared slices were compatible with optical clearing, allowing whole-mount staining of neuronal (TUJ-1) and progenitor (SOX2) markers.
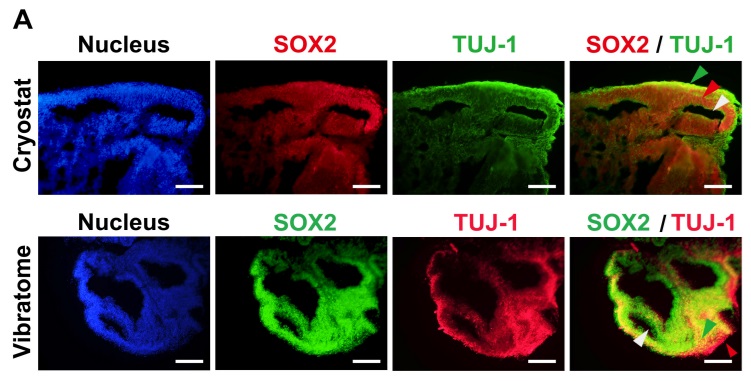
Fig. 1. Characterization of human embryonic stem cell (hESC)- and human induced pluripotent stem cell (hiPSC)-derived brain organoids. (A) 2D immunocytochemistry staining of the early neuron marker TUJ-1 and the neural progenitor marker SOX2 in hPSC-derived organoids. A Cryostat (upper) and Vibratome (lower) were used for tissue sectioning. Scale bar=100 μm (upper panels) and 200 μm (lower panels). Green arrow indicates cortical plate, red arrow indicates ventricular zone, and white arrow indicates ventricle (Cryostat sample). Green arrow indicates ventricular zone, red arrow indicates cortical plate, and white arrow indicates ventricle (Vibratome sample).
Fagerlund et al. applied vibratome slicing to live organoids for ex vivo electrophysiological studies assessing the impact of microglia-like cells on neuronal maturation.
Preparation: Organoids (107–213 days in vitro) embedded in 2% low-melting-point agarose and sliced in ice-cold, carbogenated artificial cerebrospinal fluid (aCSF).
Sectioning Parameters:
Whole-cell patch clamp: 350 µm slices.
Multielectrode array (MEA): 500 µm slices to preserve network connectivity.
Purpose: Maintain cell viability and functional synaptic circuits during recording.
Outcome: Vibratome-sliced preparations revealed that microglia-containing organoids displayed:
Sustained repetitive neuronal firing (vs. single spiking in controls).
Greater diversity of voltage-gated currents, including T-type Ca²⁺ currents.
Presence of excitatory postsynaptic currents (EPSCs) and enhanced gamma-band oscillations upon NMDA challenge.
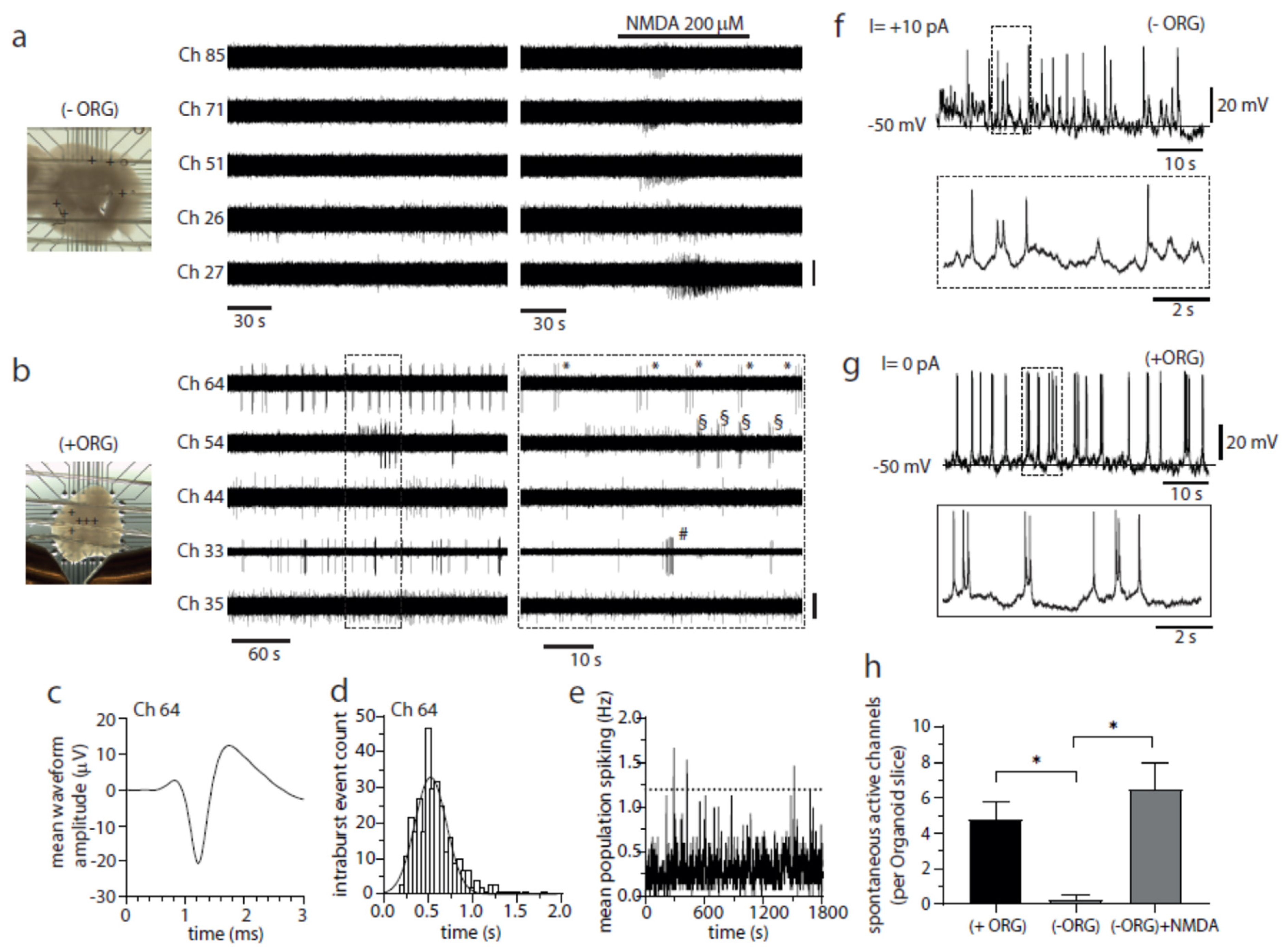
Fig.2. Incorporated IBA1+ population promotes neuronal bursting and network activity in cerebral organoids. (a) Image of an (−)ORG slice recorded with a 3D 8 × 8 MEA (left) and raw voltage recordings of spiking activity from five representative electrodes (depicted as + symbols on image) before and after NMDA perfusion (middle and right panels). Only channel 26 exhibited activity in baseline conditions (one active electrode detected from 4 organoid slices), while NMDA perfusion effectively and quickly elicited a brief burst of activity on all five channels (bar, 20 µV). (b) As described above in a but for a (+)ORG slice. The regular bursting activity of 3–7 spikes was detected in three electrodes (33, 54, 64) (twenty-four active electrodes detected from 5 -organoid slices). Symbols (*, # and §) mark individual bursts in each different channel (bar, 20 µV, except for Ch 33, 40 µV).
While both studies relied on vibratome sectioning, their applications diverged:
Hong et al. prioritised preservation of morphology for immunohistochemistry, using relatively thick slices from fixed tissue to retain fine cytoarchitectural detail.
Fagerlund et al. optimised slice viability for electrophysiology, balancing slice thickness to preserve both single-cell access and network activity.
In both cases, the vibratome provided superior preservation of structural integrity compared to cryostat sectioning — either by avoiding freezing artefacts (Hong) or by minimising shear damage to living tissue (Fagerlund). This underscores the vibratome’s versatility across fixed and live tissue contexts in cerebral organoid research.
Hong, Y.J., Lee, S.B., Choi, J., Yoon, S.H., & Do, J.T. (2022). A Simple Method for Generating Cerebral Organoids from Human Pluripotent Stem Cells. International Journal of Stem Cells, 15(1), 95–103. https://doi.org/10.15283/ijsc21195
Fagerlund, I., Dougalis, A., Shakirzyanova, A., Gómez-Budia, M., Pelkonen, A., Konttinen, H., Ohtonen, S., Fazaludeen, M.F., Koskuvi, M., Kuusisto, J., et al. (2022). Microglia-like Cells Promote Neuronal Functions in Cerebral Organoids. Cells, 11(1), 124. https://doi.org/10.3390/cells11010124